A Quick Summary of the COVID-19 Literature So Far
6 min read
What your doctor is reading on Medscape.com:
MAY 18, 2020 — Since March 25, two HIV clinical fellows, Eric Meyerowitz, MD, and Aaron Richterman, MD, MPH, have recorded a biweekly deep dive into the most compelling COVID-19 data. Their presentations have become “must-see” TV (or rather, YouTube) for those trying to make sense of all the pandemic-related literature.
Medscape asked them to summarize what they’ve learned so far, ahead of their next update, scheduled for May 19.
Below are key takeaways from our fourth update, covering April 23 to May 5, during which more than 3000 COVID-related papers were published on PubMed and another 766 were released in preprint form:
-
Remdesivir: A small, underpowered study in China found no difference in 28-day clinical improvement or mortality, in contrast to as-yet unpublished data from a larger NIAID study.
-
The benefit of IL-6 inhibitor therapy is unknown; multiple randomized controlled trials (RCTs) are ongoing.
-
Early studies suggested that viral RNA was rarely found in the blood. Now viremia/RNAemia with extrapulmonary infection is becoming more characterized, but it’s still not clear whether it represents systemic infection with infectious virus.
-
It’s currently unknown what proportion of patients have a viremic phase of illness.
-
The association between thrombosis and COVID-19 is becoming clearer, but the benefit of changing evidence-based anticoagulation strategies is unknown.
-
Structural inequities around racism and impoverishment are associated with differential outcomes, and more data are urgently needed.
-
Nonpharmaceutical interventions are important for epidemic control and economic recovery.
COVID-19 Therapy Randomized Trials
We summarized the major RCTs on COVID-19 therapeutics for which we have full papers (some in preprint form) (Table 1).
Table 1. Major COVID-19 Randomized Treatment Trials to Date
|
Ref
|
Drug Tested
|
Total N
|
Outcome
|
Notes/Limitations
|
|
|
Lopinavir/ritonavir
|
199
|
No difference
|
Clinical improvement, mortality, and percentage of patients with detectable viral RNA similar; median of 13 days from illness onset to randomization
|
|
|
Favipiravir versus umifenovir
|
240
|
For patients with moderate COVID, favipravir led to faster day 7 clinical recovery and resolution of fevers but no difference in need for mechanical ventilation
|
Preprint report
|
|
|
Hydroxychloroquine
|
150
|
No difference
|
Primary outcome of viral clearance at 28 days may not be a good endpoint; randomized late in illness course
|
|
|
Lopinavir/ritonavir versus umifenovir versus control
|
86
|
No difference for primary or secondary outcomes
|
Randomized 2:2:1; open-label trial with relatively small N; primary endpoint was time to negative RT-PCR, which may not be meaningful
|
|
|
Remdesivir
|
237
|
No difference in primary outcome; trend toward mortality improvement for those started within 10 days of symptom onset
|
Outbreak controlled in Wuhan before prespecified enrollment goal could be met: underpowered; majority of patients received steroids in this cohort
|
Continued
We also discussed additional therapeutic data from press releases (Table 2).
Table 2. Preliminary Data on COVID-19 Randomized Treatment Trials
|
Ref
|
Drug Tested
|
Total N
|
Outcome
|
Notes/Limitations
|
|
|
Remdesivir
|
1063
|
Remdesivir group: 31% faster recovery compared with placebo; trend toward improved mortality
|
Preliminary report of findings based on press release; formal report pending peer review
|
|
|
Remdesivir
|
397
|
Similar improvement in severe-disease patients who received 5 or 10 days of remdesivir
|
Industry-sponsored trial (Gilead); no placebo arm
|
|
|
Tocilizumab
|
129
|
Primary outcome was need for mechanical ventilation or death; “A significantly lower proportion of patients reached the primary outcome in the tocilizumab arm.“
|
Open-label study (no placebo); preliminary report of findings based on press release; formal report pending peer review
|
|
|
Sarilumab
|
457
|
“Negative trends” in severe-disease group in phase 2, with “positive trends” in critical group
|
Industry-sponsored trial (Regeneron/Sanofi); phase 3 trial continuing with sarilumab 400 mg versus placebo in critical group only
|
SARS-CoV-2 Transmission
The table below summarizes the key transmission findings.
Table 3. Key Findings in Mode of Transmission
|
Source
|
RNA Detected?
|
Live Virus?
|
Mode of Transmission and Evidence
|
|
|
Nasopharynx (1)
|
Yes
|
Yes
|
Droplet confirmed
|
Direct contact suspected
|
|
Sputum (1) (2)
|
Yes
|
Yes
|
Airborne likely in some circumstances
|
|
|
Saliva (3)
|
Yes
|
Yes
|
Direct contact suspected as above
|
|
|
Stool (4) (5)
|
Yes
|
Yes
|
No evidence fecal-oral to date: Macaques challenged with intragastric SARS-CoV-2 were not infected (however, direct inoculation in oral mucosa suspected)
|
|
|
Blood (6) (7)
|
Yes
|
No
|
No confirmed bloodborne transmissions to date
|
|
|
Conjunctiva (8) (9)
|
Yes
|
Yes
|
Macaques with corneal inoculation develop infection
|
|
|
Vertical
|
Yes
|
N/A
|
Several cases of fetal IgM, 1 case of neonate with RNA at 16 hours (10) (11); additionally, multiple reports of placental infection (12)
|
|
|
Semen/vaginal fluids
|
Yes
|
|
SARS-CoV-2 RNA has been detected in semen, including after recovery (13); most reports have not found virus in vaginal fluids, but there is a signal report with positive vaginal swabs (14)
|
|
|
Urine (15)
|
Yes
|
Yes
|
|
|
|
Cats (16)
|
Yes
|
Yes
|
Cats can transmit SARS-CoV-2 between each other
|
|
The Figure below illustrates what we know about the clinical course of the disease
Continued
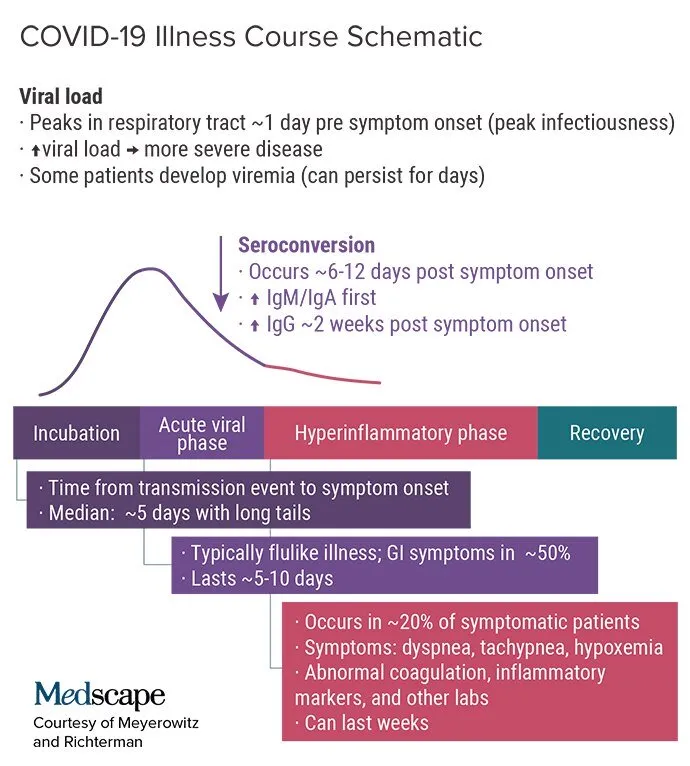
Here are some key points from our first three presentations that are still valid.
Viral Shedding: Key Points
-
Nasopharyngeal viral load peaks around 1 day prior to symptom onset, correlating to peak time of infectiousness.
-
Saliva may become an important sampling site for diagnosis.
-
SARS-CoV-2 is a descending infection; in later disease, viral loads are higher in the lower respiratory tract (especially in severe/critical illness).
-
In mild cases, live virus is isolated up to day 8 after symptom onset.
-
There can be prolonged shedding of viral RNA lasting many weeks, particularly after critical illness. Correlation with infectiousness is unknown.
-
Studies differ on whether severity of illness correlates with viral load.
-
In some cases, viral RNA has also been identified in the stool, blood, conjunctiva, urine, cerebrospinal fluid, and pleural fluid.
SARS-CoV-2 Seroprevalence
-
Studies with (near) universal screening of various populations are increasingly available, finding a wide range of asymptomatic people with positive RT-PCR tests.
-
Varying rates relate to local stage of epidemic, population and sampling, and mitigation strategies in place.
-
Some asymptomatic people are likely to be presymptomatic given the variable and sometimes lengthy incubation period.
Viral Entry: Key Points
-
ACE2 is an important receptor for viral cellular entry.
-
TMPRSS2 primes the S protein and allows for efficient cellular entry.
-
An interaction between SARS-CoV-2 and CD147 may facilitate invasion.
-
Many unresolved questions remain regarding the exact role of CD147 in viral entry. Does it directly interact with the S protein or is the interaction mediated by CypA and the N protein, as was found for SARS-CoV?
What Else Have We Learned?
Key observations from our first three updates that are still relevant:
-
Peak infectiousness is probably 1 day prior to symptom onset.
-
In the absence of therapy/vaccine, intermittent social distancing is likely to be needed for years to avoid overwhelming critical care capacity.
-
Emerging pathologic correlates of clinical presentations:
-
Multiple mechanisms of cardiac injury
-
Virus can cause systemic infection
-
Viral endotheliitis and possible complement activation as cause of micro/macro thromboses
-
-
Obesity is a risk factor for severity of disease. -
Hypercoagulability is a key feature of the disease.
-
Age-based sheltering is unlikely to be effective without social distancing.
-
Epidemic control is feasible with contact tracing if minimal delay is achieved.
-
Asymptomatic/presymptomatic transmission is substantial.
-
The incubation period is highly variable (median, 5 days).
Continued
For full details, please see our original presentations—part 1, part 2, and part 3—and be sure to tune in for part 5 on May 19.
Follow Drs Meyerowitz and Richterman on Twitter: @EricMeyerowitz and @AaronRichterman
‘);
} else
// If we match both our test Topic Ids and Buisness Ref we want to place the ad in the middle of page 1
if($.inArray(window.s_topic, moveAdTopicIds) > -1 && $.inArray(window.s_business_reference, moveAdBuisRef) > -1)
// The logic below reads count all nodes in page 1. Exclude the footer,ol,ul and table elements. Use the varible
// moveAdAfter to know which node to place the Ad container after.
window.placeAd = function(pn)
var nodeTags = [‘p’, ‘h3′,’aside’, ‘ul’],
nodes,
target;
nodes = $(‘.article-page:nth-child(‘ + pn + ‘)’).find(nodeTags.join()).not(‘p:empty’).not(‘footer *’).not(‘ol *, ul *, table *’);
//target = nodes.eq(Math.floor(nodes.length / 2));
target = nodes.eq(moveAdAfter);
$(”).insertAfter(target);
// Currently passing in 1 to move the Ad in to page 1
window.placeAd(1);
else
// This is the default location on the bottom of page 1
$(‘.article-page:nth-child(1)’).append(”);
})();
$(function()
// Create a new conatiner where we will make our lazy load Ad call if the reach the footer section of the article
$(‘.main-container-3’).prepend(”);
);
Pagination





