Parkinson’s Drug Eyed as Treatment for Severe Macular Degeneration
4 min read 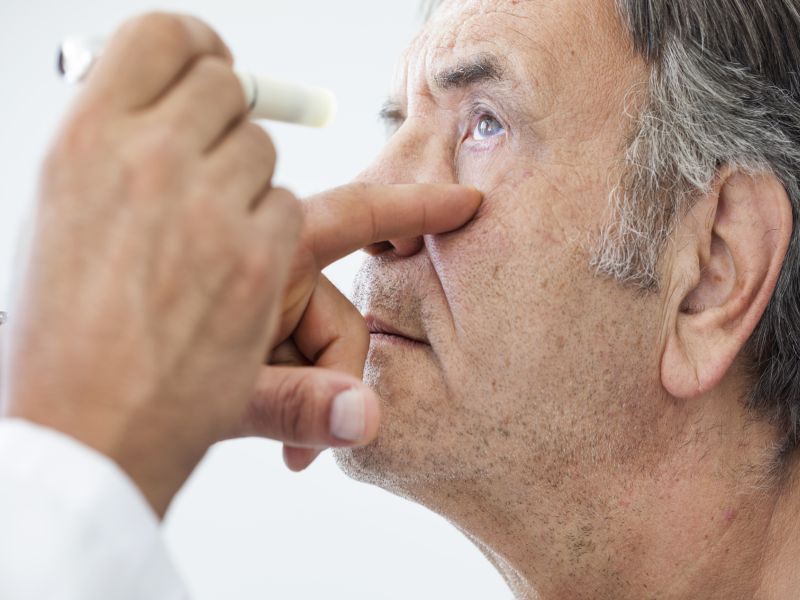 By Serena McNiff
By Serena McNiff
HealthDay Reporter
WEDNESDAY, Sept. sixteen, 2020 (HealthDay Information)
A drug very long employed to treat Parkinson’s disorder may well gain patients with a intense type of age-associated macular degeneration (AMD), a little scientific demo implies.
Just one of the leading leads to of vision loss in older people is a ailment identified as dry macular degeneration. Much more than fifteen% of Us citizens more than age 70 have AMD, and 10% to fifteen% of these conditions go on to establish the more intense wet macular degeneration, which can trigger swift and total vision loss.
Normally, wet AMD is treated with injections of treatment into the eye. Most people will need numerous per 12 months to preserve the disorder from progressing.
But this little, early-phase scientific demo implies an alternate may well be on the horizon: the leading drug employed to treat Parkinson’s disorder, identified as levodopa.
The demo was an outgrowth of a 2016 examine that found Parkinson’s patients who took levodopa had been fewer probable to establish macular degeneration.
“The examine found a relationship in between taking levodopa and macular regeneration,” stated Dr. Robert Snyder, a professor of ophthalmology at the College of Arizona, in Tucson. “It delayed the onset of both dry and wet macular degeneration, and minimized the odds of acquiring wet macular degeneration.”
Macular degeneration has an effect on the macula, element of the eye that will allow you to see good detail. Soaked AMD happens when abnormal blood vessels mature beneath the macula frequently, these blood vessels leak blood and fluid, triggering immediate injury.
Snyder and two colleagues began a scientific demo in 2017 to discover whether or not levodopa may aid reduce both varieties of AMD.
20 patients recently identified with AMD took element in the 1st demo. Each and every was presented a little day-to-day dose of levodopa for one particular thirty day period.
An eye medical doctor evaluated them weekly to figure out whether or not they also required an eye injection. Given that the demo was primarily based on preliminary analysis, the authors desired patients to obtain injections if needed, to assure that their ailment would not worsen if the levodopa was ineffective.
“Rather of injecting them, which would have been the conventional of treatment, we treated them with levodopa and followed them weekly to make sure they failed to get worse,” Snyder stated. “And if they did get worse, we despatched them back again for an injection.”
Right after one particular thirty day period, all contributors joined eleven new enrollees in a second demo to appraise levodopa’s protection and efficiency at unique doses.
When many contributors required an injection throughout the demo, they necessary fewer pictures than would generally be presented throughout a one particular-thirty day period period of time. Having levodopa also seemed to hold off the will need for an injection, the examine found.
The authors noted that taking levodopa enhanced participants’ vision over-all. It also appreciably diminished the buildup of fluid in the eye.
The drug was revealed to be harmless and very well-tolerated, the researchers stated. People who skilled side results linked with the treatment, these types of as nausea and blurred vision, had been put on a decreased dose.
But this type of “open up-label demo” has some limitations. There was no place of comparison, these types of as a placebo all contributors been given levodopa. And researchers and contributors all understood what treatment was administered, likely introducing bias to the results.
Dr. Raj Maturi, scientific spokesman for the American Academy of Ophthalmology, stated confirming the drug’s protection and efficiency will need a more substantial, more strong scientific demo.
Maturi also expressed worry about probable side results of levodopa, particularly presented the age of the inhabitants that is affected by macular degeneration.
“You might be chatting about a inhabitants of their 70s and 80s — they currently have other matters heading on,” Maturi stated. “An supplemental oral systemic drug that they will choose for the relaxation of their life can appreciably influence their top quality of life. I am normally involved about the side effect profiles of oral drugs that have to be taken for a very long period of time of time.”
When the second element of the demo is ongoing, early results had been printed online not long ago in The American Journal of Medicine. Snyder stated a more substantial examine is forthcoming.
“We felt really strongly that we experienced a constructive effect and experienced a proof of principle to go ahead with a more substantial, placebo-controlled scientific demo,” he stated. “That’s heading to be our following phase.”

Copyright © 2020 HealthDay. All legal rights reserved.
SLIDESHOW
Dementia, Alzheimer’s Illness, and Growing old Brains
See Slideshow
References
Resources: Robert Snyder, MD, PhD, office head, ophthalmology, and professor, biomedical engineering, College of Arizona, Tucson, and president/founder, Snyder Biomedical Corporation, Tucson Raj Maturi, MD, scientific spokesman, American Academy of Ophthalmology, and scientific associate professor, Indiana College, Indianapolis The American Journal of Medicine, July 3, 2020, online






