Trending Clinical Topic: Remdesivir
3 min readWhat your physician is looking through on Medscape.com:
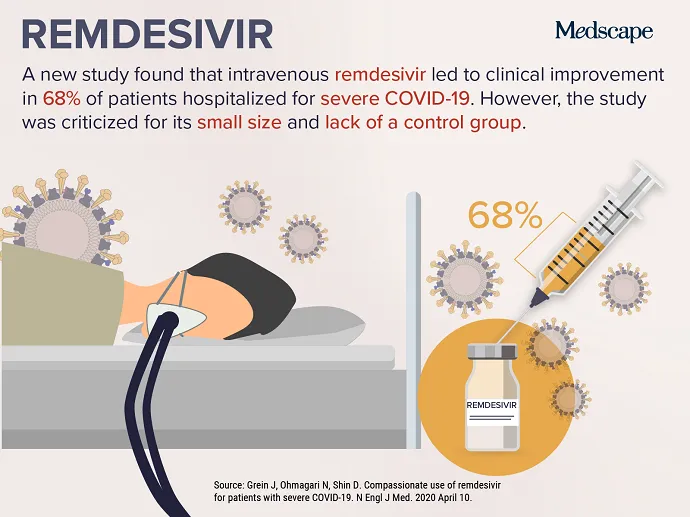
APRIL 24, 2020 — As the COVID-19 pandemic carries on, endeavours to determine a safe and sound and helpful remedy have intensified, top to this week’s best trending medical subject. New data on the investigational antiviral drug remdesivir were published April 10 in the New England Journal of Medication . Far more than two thirds of severely sick patients with COVID-19 who have been supplied remdesivir for “compassionate use” enhanced immediately after getting the treatment. Of the fifty three patients included in the analyze, 22 have been in the United States, 22 have been in Europe or Canada, and 9 have been in Japan. Individuals obtained a 10-working day class of remdesivir, consisting of two hundred mg administered intravenously on working day 1, adopted by one hundred mg daily for the remaining 9 times of treatment method.
Although some have been inspired by the outcomes, some others warned that limitations in the analyze make decoding the data tough. Josh Farkas, MD, an intensivist from Vermont, provided 11 good reasons why the analyze “reveals absolutely nothing” and identified as the paper “scorching garbage” on Twitter. Other doctors lamented the erosion of the peer-critique course of action throughout the COVID-19 pandemic. The absence of a control team and a compact affected individual sample dimension have been frequently cited as considerable concerns.
In a letter to Gilead, the enterprise guiding remdesivir, John Mandrola, MD, calls for blinding and a placebo arm to be additional to long run trials. Although he applauds the endeavours manufactured as a result far, Mandrola reiterates that these additions would make the results extra trusted, as “they would be absolutely free from the possible biases of clinicians—who, out of the need to have an obtainable treatment method, may possibly consciously or unconsciously make or delay choices to minimize oxygen and stay clear of mechanical air flow in patients in the remdesivir arm.”
At this place, an pro panel of the Infectious Health conditions Culture of The usa (IDSA) states evidence is insufficient to suggest any possible pharmacologic therapies for program use in patients with COVID-19. The effort to validate a treatment method technique is large and international. The Globe Health Organization has identified a listing of “promising candidates” for COVID-19 treatment method, such as remdesivir, lopinavir-ritonavir, immunotherapies, and convalescent sera. Around sixty trials involving these therapies have been planned, are recruiting, or have already started. As the outcomes of individuals trials turn out to be obtainable, the medicines researched will obtain huge focus, just as remdesivir did this week.
‘)
} else
// If we match both of those our exam Subject Ids and Buisness Ref we want to place the advertisement in the center of site 1
if($.inArray(window.s_subject, moveAdTopicIds) > -1 && $.inArray(window.s_business enterprise_reference, moveAdBuisRef) > -1)
// The logic beneath reads depend all nodes in site 1. Exclude the footer,ol,ul and desk components. Use the varible
// moveAdAfter to know which node to place the Ad container immediately after.
window.placeAd = function(pn)
var nodeTags = [‘p’, ‘h3′,’aside’, ‘ul’],
nodes,
concentrate on
nodes = $(‘.article-site:nth-youngster(‘ + pn + ‘)’).uncover(nodeTags.be part of()).not(‘p:empty’).not(‘footer *’).not(‘ol *, ul *, desk *’)
//concentrate on = nodes.eq(Math.ground(nodes.length / two))
concentrate on = nodes.eq(moveAdAfter)
$(”).insertAfter(concentrate on)
// Now passing in 1 to go the Ad in to site 1
window.placeAd(1)
else
// This is the default site on the base of site 1
$(‘.article-site:nth-youngster(1)’).append(”)
})()
$(function()
// Generate a new conatiner where by we will make our lazy load Ad phone if the reach the footer part of the article
$(‘.most important-container-3’).prepend(”)
)






